Colorectal Caner
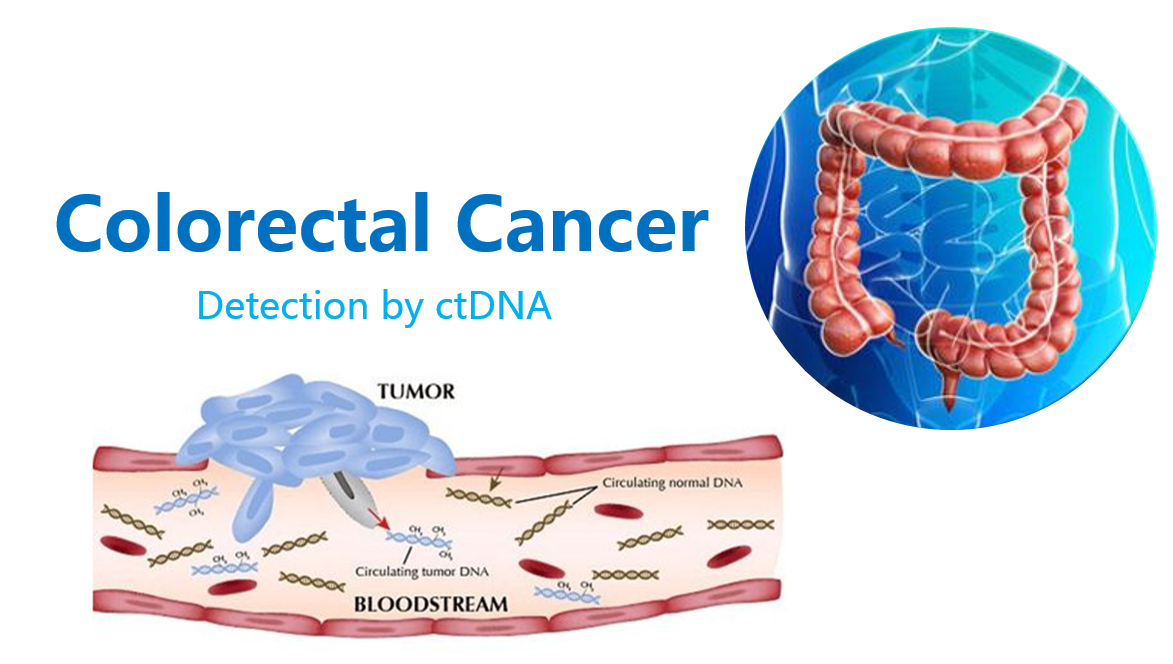
Colorectal cancer is currently the third most common malignant cancer in China. About 25% colorectal cancer patients have developed local or distant metastases at the time of initial diagnosis. The 5-year survival rate for them is as low as 12%. However, if the patients are found at early-stage of colorectal cancer, the 5-year survival rate is over 50% after receiving treatment. Therefore, effective early diagnosis and selection of appropriate drug therapy are vitally important for patients with colorectal cancer.
Liquid biopsy helps the diagnosis of cancer and other diseases through testing of such body fluids as blood or saliva. Liquid biopsy uses circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), circulating tumor RNA (ctRNA) and exosome (carrying cell-derived proteins, lipids, DNA, RNA and so on) as a biomarker for diagnosis of cancer. Compared with tissue biopsy, liquid biopsy is non-invasive, allows repeat sampling of tumor and thus revealing the “whole picture” of tumor. It largely compensates for the shortcomings of tissue biopsy.
Early Diagnosis of Colorectal Cancer
In 2008, Lofton-Day and others found that Septin9 DNA methylation is associated with the occurrence of colorectal cancer. Based on this finding, they began to apply plasma Septin9 test to early diagnosis of colorectal cancer. Later, a number of studies at home and abroad confirmed that the sensitivity of the test is greater than 79%, and the specificity of the test is greater than 85%. Now plasma mSEPT9 test has been clinically applied as a part of colorectal cancer screening program. Through the combination of plasma mSEPT9 test, fecal occult blood test, plasma protein marker detection, endoscopy, tissue biopsy and other techniques, effective early diagnosis of colorectal cancer can be achieved.
Postoperative Prognosis and Residual Disease Monitoring
The amount of ctDNA is correlated with tumor burden and staging. As a new biomarker, ctDNA is of great help to real-time, dynamic and non-invasive clinical detection of changes in tumor. Results of detection of specific gene mutation sites, abnormal methylation and copy number variation level in ctDNA can be used as reference for assessment of tumor treatment effect and residual disease monitoring.
Compared with ctDNA, CTCs have been used in the postoperative prognosis and recurrence monitoring of colorectal cancer for a long time. Studies have shown that when a patient has over 3 CTCs per 7.5 mL blood detected, his 5-year survival rate is low and the treatment effect is relatively poor; if the number of CTCs decreased after treatment, the survival rate and prognostic effect are better. The number of CTCs captured using specific markers before or after surgery is used as a guidance for postoperative prognosis evaluation.
Targeted Therapy of Colorectal Cancer
Anti-EGFRs targeted therapy is currently used in first-line treatment of mCRC. Drug resistance in colorectal cancer caused by anti-EGFRs targeted therapy is related to EGFR downstream pathway gene mutation. ctDNA carries genetic information of tumor cells. Detection of peripheral blood related genes (such as KRAS, NRAS, etc.) mutation abundance can provide evidence for the occurrence of clinical resistance. Through quantitative analysis of mutation in specific genes of ctDNA in colorectal cancer patients, the dynamic process of tumor evolution in drug therapy can be observed, thus providing the basis for targeted treatment of colorectal cancer.
Studies have shown that mutations in KRAS were detected in CTCs in patients with mCRC and 30% of such patients receiving targeted drug therapy developed secondary KRAS mutations, which indicates that the monitoring of molecular changes in CTCs in peripheral blood can be applied to targeted drug therapy.
We offer detection of colorectal cancer at the following sites:
|
Part of the genes for detection |
APC ARIDIA ALK ARAF BRAF BRCA1 BRCA2 CDKN2A EGFR ERBB2 FGFR1 KRAS MAP2K1 MYC MET NRAS HER2 PIK3CA |
Sample requirements:
Blood: 10 ml whole blood, stored and transported in Streck Cell-Free DNA BCT tubes
Plasma and blood cell: After the blood was collected, collect the supernatant and sediment by centrifuging (4℃, 3000g, 10 min) the whole blood, then centrifuge (4℃, 16000g, 10 min) the supernatant to get new supernatant.
Qualified patients for this test:
1. Colorectal cancer patients who expect to adopt targeted drug therapy.
2. Colorectal cancer patients who wish to replace a poor therapy with an effective new one.
3. Colorectal cancer patients who need new therapy because of drug resistance, recurrence or metastasis of the cancer.
4. Colorectal cancer patients who need the assessment of postoperative prognosis and recurrent risk.
- 上一篇:Breast Caner
- 下一篇:Lung Caner
