Breast Caner
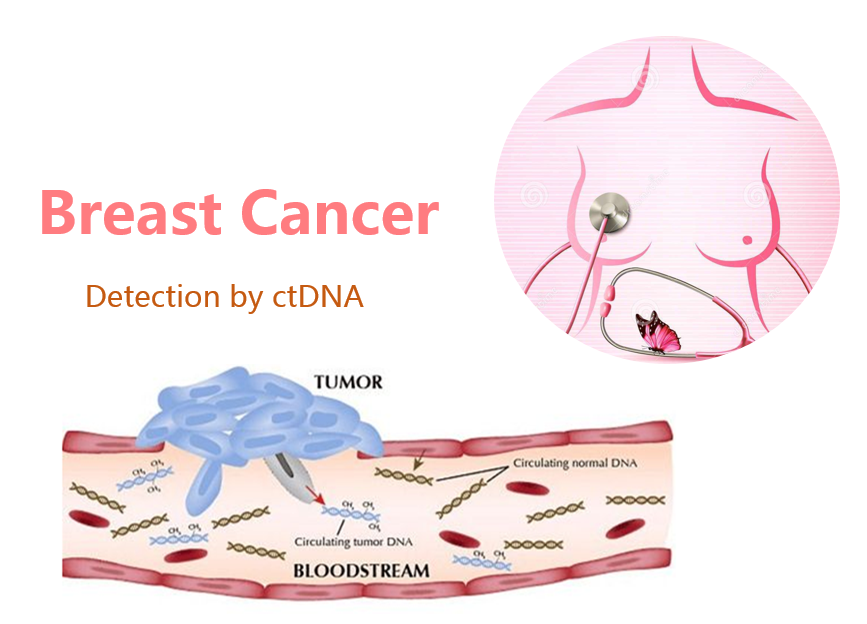
With the development of high-throughput sequencing technology, the application of circulating tumor DNA (ctDNA) in the early detection of breast cancer has drawn increasing attention. A large number of studies at home and abroad have shown that ctDNA detection technology has offered great application value in monitoring tumor load, effective treatment prediction, early diagnosis and prognosis of tumor. Today, as individualized precision has become the developemnt trend of breast cancer treatment, ctDNA detection can be used to provide patients with more accurate diagnosis and to guide clinical treatment. Omigen offers breast cancer patients comprehensive clinical services including: detection of genes related to breast cancer, in-depth interpretation of genetic mutation, latest targeted drug and chemotherapeutic drug information, genetic risk analysis and individualized medication guidance.
We offer detection of breast cancer at the following sites:
|
Part of the genes for detection |
ABCB1 BRCA1 BRCA2 BRAF CDK4 CDK6 CYP19A1 CDH1 CCND1 EGFR ERBB3 ESR1 HER2 TSC1 PIK3CA NTRK1 TP53 PTEN |
Qualified patients for this test:
- Breast cancer patients who expect to adopt targeted drug therapy.
- Breast cancer patients who need targeted treatment and important risk assessment for relatives through screening for hereditary breast cancer.
- Breast cancer patients who need chemotherapy and assessment of toxic side effects of chemotherapeutic drugs.
- Healthy people: all people who are concerned about breast cancer.
Clinical significance:
Applicability in early screening, personalized medication, efficacy evaluation, recurrence monitoring and so forth.
Sample requirements:
Blood: 10 mL whole blood, stored and transported in Streck Cell-Free DNA BCT tubes
Plasma and blood cell: After the blood was collected, collect the supernatant and sediment by centrifuging (4℃, 3000g, 10 min) the whole blood, then centrifuge (4℃, 16000g, 10 min) the supernatant to get new supernatant.
- 上一篇:FAQ
- 下一篇:Colorectal Caner
